The main organic substances of the cell are proteins, carbohydrates, nucleic acids and lipids.
Carbohydrates. In the cell are represented by monosaccharides, disaccharides and polysaccharides.
Monosaccharides - colorless solid crystalline substances, readily soluble in water, usually sweet in taste. Monosaccharides include glucose, fructose, ribose, deoxyribose, and others. There is a lot of glucose and fructose in honey and fruits. Ribose and deoxyribose are part of the nucleic acids.
Complex and large molecules polysaccharides (starch, cellulose, glycogen) consist of many interconnected residues of monosaccharide molecules. Polysaccharides such as starch, cellulose, glycogen are composed of glucose molecules linked together, the number of which is variable and can range from hundreds of thousands to millions. Therefore, the general formula for starch, glycogen and cellulose looks like this: (C 6 H 10 O 5) n.
When two glucose molecules combine, one water molecule is split off. Symbol n means that the number of glucose molecules in starch, glycogen and cellulose molecules can vary. Cellulose has a linear structure, while starch and glycogen are branched.
The difference between cellulose and starch molecules is also that the number n cellulose has more. One starch macromolecule contains from several hundred to several thousand units, and a cellulose molecule contains over 10,000 units. Cellulose forms fibers that give the plant its rigidity and strength. Thus, cellulose fiber is stronger than steel wire of the same diameter.
Lipids (from Greek - fat). Fat molecules are formed by residues of trihydric alcohol (glycerol) and residues of fatty acid molecules. The main property of lipids is hydrophobicity.
Features of the structure of molecules of carbohydrates and lipids determine their functions in the cell.
Functions of carbohydrates and lipids in the cell.
1. Supply of nutrients in the cell.
The cells of potato tubers and rhizomes of many plants are rich in carbohydrates. Glycogen accumulates in liver and muscle cells. When the body needs energy, glycogen molecules are broken down into readily soluble glucose molecules. Fat reserves are contained in the cells of adipose tissue of birds and mammals, the seeds of some plants. In chordates, fat stores are stored under the skin and serve to protect the body from hypothermia and mechanical damage. So, whales, walruses, seals, penguins are protected from hypothermia by powerful fat deposits. In a whale, for example, the layer of subcutaneous fat reaches 1 m.
2. Energy. Molecules of carbohydrates and fats are oxidized in cells to carbon dioxide and water, and the energy released during this is used for vital processes.
3. Structural. Carbohydrates and lipids are found in various parts and organelles of the cell. So, the cell walls of plants are built from cellulose. The wood contains from 40 to 60% cellulose. Lipids are an essential component of the cell membrane.
Proteins.
Functions of proteins in the cell:
1. Catalytic... Catalyst proteins accelerate chemical reactions in the cell. So catalase increases the rate of decomposition of hydrogen peroxide (H 2 O 2) by 10 11 times
2. Regulatory.For example, the protein insulin regulates blood sugar.
3. Structural. Protein molecules are part of all cell membranes. Collagen protein molecules form the basis of cartilage and tendons. Protein consists of hair, wool, nails, horns, hooves, scales, feathers, cobwebs.
4. Motor. Some proteins (actin, myosin) are capable of causing muscle fibers to contract.
5. Protective.... Antibodies formed in vertebrates are proteins that neutralize foreign substances entering the body. The protein fibrinogen is involved in blood clotting.
6. Transport.... For example, the blood protein hemoglobin, which is part of erythrocytes, forms fragile compounds with oxygen in the lungs and delivers it to all cells of the body.
7. Storing. accumulating, for example, in plant seeds.
8. Energy.With a lack of polysaccharides and lipids, proteins can perform an energy function. When protein molecules are oxidized in the cell, energy is released in about the same amount as when carbohydrates are oxidized.
Nucleic acids.
Nucleic acids were discovered in the second half of the 19th century. by the Swiss biochemist F. Mischer, who isolated a substance with a high content of nitrogen and phosphorus from the nuclei of cells and called it "nuclein" (from Latin nucleos - nucleus).
There are two types of nucleic acids - DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). Nucleic acids, like proteins, are species specific, that is, each species has its own type of DNA.
Nucleic acid molecules are very long chains of many hundreds or even millions of nucleotides. Any nucleic acid contains only four types of nucleotides. The functions of nucleic acid molecules depend on the number in the chain and the sequence of the compound in the nucleotide molecule.
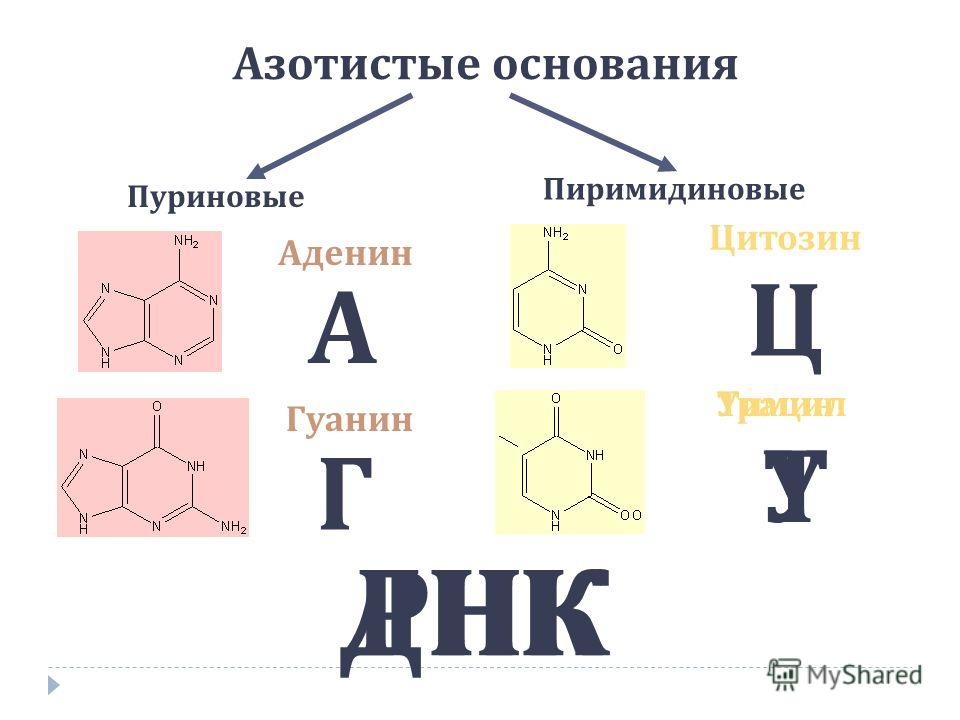
Each nucleotide consists of three components: a nitrogenous base, a carbohydrate, and phosphoric acid. Each DNA nucleotide contains one of four types of nitrogenous bases (adenine - A, thymine - T, guanine - G, or cytosine - C), as well as a deoxyribose carbohydrate and a phosphoric acid residue.
In 1953, the American biologist J. Watson and the English physicist F. Crick created a model of the structure of the DNA molecule. Scientists have found that each DNA molecule consists of two chains, linked together and spirally twisted. It looks like a double helix. In each strand, the four types of nucleotides alternate in sequence.
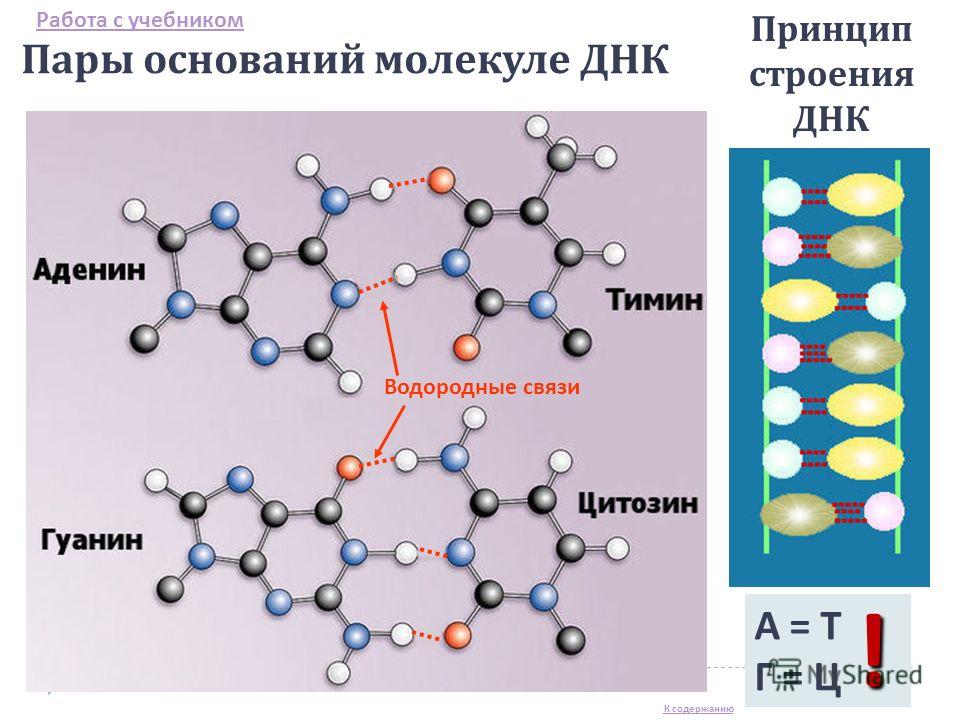
The nucleotide composition of DNA differs in different types bacteria, fungi, plants, animals. But it does not change with age, depends little on changes in the environment. The nucleotides are paired, that is, the number of adenyl nucleotides in any DNA molecule is equal to the number of thymidyl nucleotides (AT), and the number of cytidyl nucleotides is equal to the number of guanyl nucleotides (C-G). This is due to the fact that the connection of two chains to each other in a DNA molecule obeys a certain rule, namely: the adenine of one chain is always linked by two hydrogen bonds only with the thymine of the other chain, and guanine is always linked by three hydrogen bonds with cytosine, that is, the nucleotide chains of one molecule DNA is complementary, complementary to each other DNA contains all bacteria, the vast majority of viruses. It is found in the nuclei of cells of animals, fungi and plants, as well as in mitochondria and chloroplasts. The nucleus of each cell of the human body contains 6.6 x 10 -12 g of DNA, and in the nucleus of the germ cells - half as much - 3.3 x 10-12 g.
Nucleic acid molecules - DNA and RNA are made up of nucleotides. The composition of DNA nucleotides includes a nitrogenous base (A, T, G, C), a deoxyribose carbohydrate, and the remainder of a phosphoric acid molecule. The DNA molecule is a double helix consisting of two strands connected by hydrogen bonds according to the principle of complementarity. The function of DNA is to store hereditary information.
An RNA molecule, unlike DNA, is usually a single chain of nucleotides that is much shorter than DNA. However, the total mass of RNA in a cell is greater than that of DNA. RNA molecules are found both in the nucleus and in the cytoplasm.
There are three main types of RNA: informational, or template, - mRNA; ribosomal - rRNA, transport - tRNA, which differ in the shape, size and function of molecules. Their main function is to participate in protein biosynthesis.
The structure of RNA molecules is shown. You can see that an RNA molecule, like a DNA molecule, consists of four types of nucleotides, three of which contain the same nitrogenous bases as DNA nucleotides (A, G, C). However, instead of the nitrogenous base of thymine, RNA contains another nitrogenous base, uracil (U). Thus, the nucleotides of the RNA molecule include nitrogenous bases: A, G, C, U. In addition, instead of the carbohydrate deoxyribose, RNA contains ribose
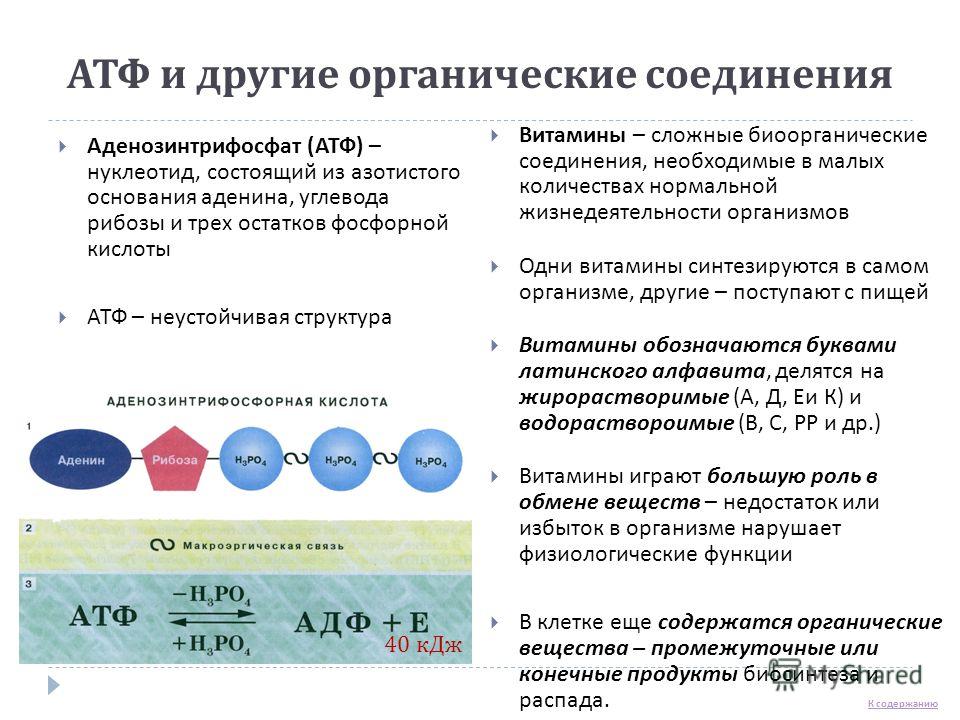
In the cells of all organisms, there are molecules of ATP - adenosine triphosphoric acid. ATP is a universal cell substance, the molecule of which has energy-rich bonds. ATP molecule is one kind of nucleotide, which, like other nucleotides, consists of three components: a nitrogenous base - adenine, a carbohydrate - ribose, but instead of one it contains three residues of phosphoric acid molecules The bonds indicated by ~ are rich in energy and are called macroergic... Each ATP molecule contains two high-energy bonds.
When the high-energy bond is broken and one molecule of phosphoric acid is cleaved with the help of enzymes, 40 kJ / mol of energy is released, while ATP is converted into ADP - adenosine diphosphoric acid. When one more phosphoric acid molecule is cleaved off, another 40 kJ / mol is released; AMP is formed - adenosine monophosphoric acid. These reactions are reversible, that is, AMP can be converted into ADP, ADP - into ATP
ATP molecules are not only broken down, but also synthesized, so their content in the cell is relatively constant. The value of ATP in the life of a cell is enormous. These molecules play a leading role in the energy metabolism necessary to ensure the vital activity of the cell and the body as a whole.
Organic substances are the basis of all living nature. Plants and animals, micro-organisms and viruses - all living things consist of a huge amount of various organic substances and a relatively small number of inorganic ones. It was carbon compounds, due to their great diversity and ability to numerous chemical transformations, that were the basis on which life arose in all its manifestations. This required very complex organic substances, the molecules of which contain chains of many thousands of atoms, i.e., polymers. Such polymers are called biopolymers.
First of all, these are proteins - carriers of life, the basis of a living cell. Complex organic polymers - proteins are mainly composed of carbon, hydrogen, oxygen, nitrogen and sulfur. Their molecules are formed by combining a very large number of simple molecules - the so-called amino acids. There are about twenty amino acids, but they combine with each other in a different combination, strictly characteristic and constant for every protein, every organ, every tissue, every type of living creature. And since the length of protein molecules can be very large - up to several tens of thousands of individual amino acids in a chain, it becomes clear how exactly proteins provide a variety of manifestations of life.
There are many different proteins.
There are supporting proteins, the purpose of which is to form the skeleton, integuments and other supports of the body. Such proteins are part of the bones, form cartilage, skin, hair, horns, hooves, feathers, fish scales. There are supporting proteins that form muscles and carry not only supporting, but also contractile functions. Muscle contraction (the most important role of this type of protein) is the conversion of part of the chemical energy of such proteins into mechanical work.
 There are blood proteins with a wide variety of functions. Blood hemoglobin, for example, serves as a carrier of oxygen throughout the body. A very large group of proteins regulates chemical reactions in organisms. These are enzymes (biological catalysts). More than seven hundred of them are known. Highly developed organisms are also able to produce protective proteins, the so-called antibodies, which are capable of precipitating and thereby neutralizing foreign substances and bodies that have entered the body from the outside.
There are blood proteins with a wide variety of functions. Blood hemoglobin, for example, serves as a carrier of oxygen throughout the body. A very large group of proteins regulates chemical reactions in organisms. These are enzymes (biological catalysts). More than seven hundred of them are known. Highly developed organisms are also able to produce protective proteins, the so-called antibodies, which are capable of precipitating and thereby neutralizing foreign substances and bodies that have entered the body from the outside.
Along with proteins, nucleic acids play an important role in life. These substances build proteins. In a living organism, metabolism always takes place. The composition of almost all of its cells is constantly renewed. Cell proteins are also renewed. But after all, for each organ, for each tissue, it is necessary to make its own specific protein, with its own unique order of amino acids in the chain. The keepers of this order are nucleic acids. These are also polymeric molecules, complexly constructed, often in the form of a double helix of atoms. Nucleic acids are a kind of templates by which organisms build their proteins. It is often figuratively said that they contain the protein synthesis code. Each protein has its own code, its own template. Nucleic acids have another function. They are templates for the nucleic acids themselves. It is a kind of "memory device" with the help of which each type of living creature transmits from generation to generation the codes for the construction of their proteins.
Supporting functions in living nature are performed not only by proteins. In plants, for example, support, skeletal substances - cellulose and lignin. These are also polymeric substances, but of a completely different type. The long chains of cellulose atoms are built from glucose molecules, a sugar group. Therefore, cellulose is referred to as polysaccharides. The structure of lignin has not yet been fully established. This is also a polymer, apparently with reticulated molecules. And in insects, chitin, also a polysaccharide, performs supporting functions.
There is a large group of substances (fats, sugars or carbohydrates) that carry and store chemical energy. They are a spare building material necessary for the formation of new cells (see the article "Chemistry of food"). A lot of organic substances in living organisms play the role of regulators of vital activity (vitamins, hormones). Some regulate the growth and division of cells, others - respiration or digestion, others - the activity of the nervous system, etc. Living organisms also contain numerous substances of the most diverse purposes: coloring, to which the world of flowers owes its beauty, pa -hooking - attracting or scaring off, protecting from external enemies, and many others. Plants and animals, even every single cell, are very complex laboratories in which thousands of organic substances arise, transform and decompose. Numerous and varied chemical reactions take place in these laboratories in a strictly defined sequence. The most complex structures are created, grow and die off ...
The world of organic substances surrounds us, we ourselves consist of them, and all living nature, with which we every second encounter, among which we live and which we constantly use, consists of organic substances.
1. Carbohydrates consist of ...
- carbon, hydrogen and oxygen
- carbon, nitrogen and hydrogen
- carbon, oxygen and nitrogen
Carbohydrates, or saccharides, is one of the main groups of organic compounds. They are part of the cells of all living organisms. Carbohydrates are made up of carbon, hydrogen and oxygen. They got their name because most of them have the same ratio of hydrogen and oxygen in a molecule as in a water molecule.
General formula of carbohydrates: Сn (Н 2 О) m. Examples include glucose - C 6 H 12 O 6 and sucrose - C 12 H 22 O 11. Other elements can also be included in carbohydrate derivatives. All carbohydrates are divided into simple, or monosaccharides, and complex, or polysaccharides... Of the monosaccharides, ribose, deoxyribose, glucose, fructose, galactose are of the greatest importance for living organisms.
Functions of carbohydrates: energetic, building, protective, storage.
2. Determine the proposed polysaccharides.
- starch, glycogen, chitin ...
- glucose, fructose, galactose
- ribose, deoxyribose
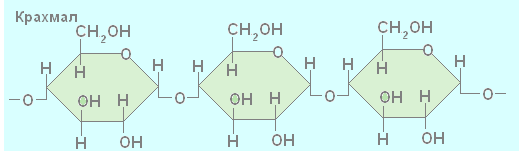
Di- and polysaccharides are formed by combining two or more monosaccharides. Disaccharides are similar in properties to monosaccharides. Both are highly water soluble and have a sweet taste. Polysaccharides are composed of a large number of monosaccharides connected by covalent bonds. These include starch, glycogen, cellulose, chitin and others.
3. Violation of the natural structure of the protein.
- denaturation
- renaturation
- degeneration
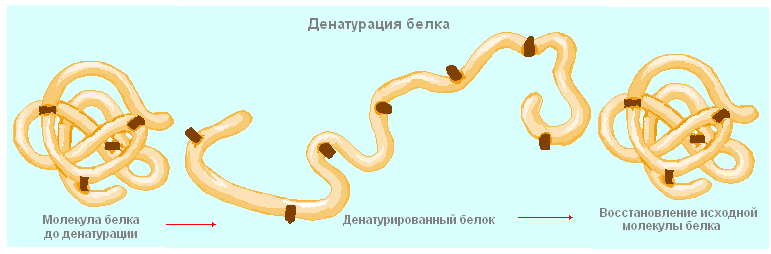
Violation of the natural structure of the protein is called denaturation... It can occur under the influence of temperature, chemicals, radiant energy, and other factors. With a weak impact, only the quaternary structure disintegrates, with a stronger one, the tertiary structure, and then the secondary one, and the protein remains in the form of a polypeptide chain. This process is partially reversible: if the primary structure is not destroyed, then the denatured protein is able to restore its structure. Thus, all the structural features of a protein macromolecule are determined by its primary structure.
4. The function due to which the acceleration of biochemical reactions in the cell occurs.
- catalytic
- enzymatic
- both answers are correct
![]() Enzymes (or biocatalysts) are protein molecules that act as biological catalysts that increase the rate of chemical reactions thousands of times. For large organic molecules to react, simple contact is not enough for them. It is necessary that the functional groups of these molecules face each other and no other molecules interfere with their interaction. The probability that the molecules themselves will orient themselves in the right way is negligible. The enzyme, on the other hand, attaches to itself both molecules in the desired position, helps us to get rid of the water film, supplies energy, removes excess parts and releases the finished reaction product. At the same time, the enzymes themselves, like other chemical catalysts, do not change as a result of past reactions and do their job over and over again. There are optimal conditions for the functioning of each enzyme. Some enzymes are active in a neutral environment, others in an acidic or alkaline environment. At temperatures above 60 ° C, most enzymes do not function.
Enzymes (or biocatalysts) are protein molecules that act as biological catalysts that increase the rate of chemical reactions thousands of times. For large organic molecules to react, simple contact is not enough for them. It is necessary that the functional groups of these molecules face each other and no other molecules interfere with their interaction. The probability that the molecules themselves will orient themselves in the right way is negligible. The enzyme, on the other hand, attaches to itself both molecules in the desired position, helps us to get rid of the water film, supplies energy, removes excess parts and releases the finished reaction product. At the same time, the enzymes themselves, like other chemical catalysts, do not change as a result of past reactions and do their job over and over again. There are optimal conditions for the functioning of each enzyme. Some enzymes are active in a neutral environment, others in an acidic or alkaline environment. At temperatures above 60 ° C, most enzymes do not function.
5. Function of contractile proteins.
- motor
- transport
- protective
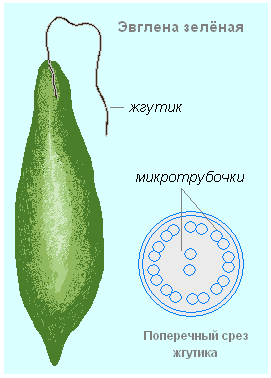 Motor the function of proteins is performed by special contractile proteins. Thanks to them, cilia and flagella move in protozoa, chromosomes move during cell division, muscles contract in multicellular organisms, and other types of movement in living organisms are improved.
Motor the function of proteins is performed by special contractile proteins. Thanks to them, cilia and flagella move in protozoa, chromosomes move during cell division, muscles contract in multicellular organisms, and other types of movement in living organisms are improved.
The flagellum of all eukaryotic cells is about 100 μm long. On the cross section, it can be seen that 9 pairs of microtubules are located along the periphery of the flagellum, and 2 microtubules are located in the center. All pairs of microtubules are interconnected. The protein that carries out this binding changes its conformation due to the energy released during the hydrolysis of ATP. This leads to the fact that pairs of microtubules begin to move relative to each other, the flagellum bends and the cell begins to move.
6. Function of proteins, due to which hemoglobin carries oxygen from the lungs to the cells of other tissues and organs.
- transport
- motor
- both answers are correct
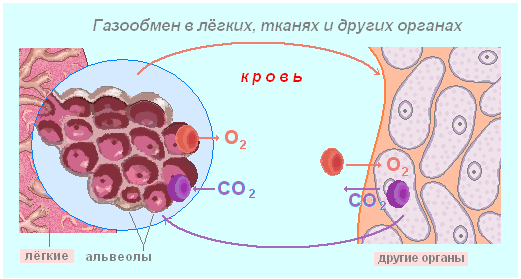
It is important transport function of proteins. So, hemoglobin carries oxygen from the lungs to the cells of other tissues and organs. In muscles, this function is performed by the protein hemoglobin. Serum proteins (albumin) promote the transfer of lipids and fatty acids, various biologically active substances. By attaching oxygen, hemoglobin from bluish color becomes scarlet. Therefore, blood, in which there is a lot of oxygen, differs in color from blood in which there is little oxygen. Transport proteins in the outer membrane of cells carry various substances from the environment into the cytoplasm.
7. Protein function, which maintains a constant concentration of substances in the blood and body cells. Participate in growth, reproduction and other vital processes.
- enzymatic
- regulatory
- transport
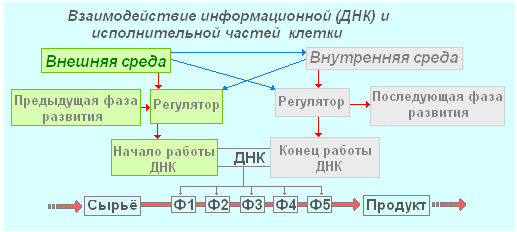 Regulatory the function is inherent in proteins - hormones. They maintain constant concentrations of substances in the blood and cells, participate in growth, reproduction and other vital processes. In the presence of a regulator substance, the reading of a certain DNA section begins. The protein produced by this gene begins a long chain of transformations of substances passing through the enzymatic complex. In the end, a regulator substance is produced, which stops reading or transfers it to another site. At the same time, it is the DNA information that determines which substances to produce, and the final product of the synthesis blocks the DNA and suspends the entire process. Another way: DNA is blocked by a substance that appears as a result of the activity of the body's control systems: nervous or humoral. Of course, there can be a large number of intermediaries in this chain. For example, there is a whole group of receptor proteins that send a control signal in response to changes in the external or internal environment.
Regulatory the function is inherent in proteins - hormones. They maintain constant concentrations of substances in the blood and cells, participate in growth, reproduction and other vital processes. In the presence of a regulator substance, the reading of a certain DNA section begins. The protein produced by this gene begins a long chain of transformations of substances passing through the enzymatic complex. In the end, a regulator substance is produced, which stops reading or transfers it to another site. At the same time, it is the DNA information that determines which substances to produce, and the final product of the synthesis blocks the DNA and suspends the entire process. Another way: DNA is blocked by a substance that appears as a result of the activity of the body's control systems: nervous or humoral. Of course, there can be a large number of intermediaries in this chain. For example, there is a whole group of receptor proteins that send a control signal in response to changes in the external or internal environment.
8. The DNA molecule contains nitrogenous bases ...
- adenine, guanine, cytosine, thymine
- adenine, guanine, leucine, thymine
- there is no right answer
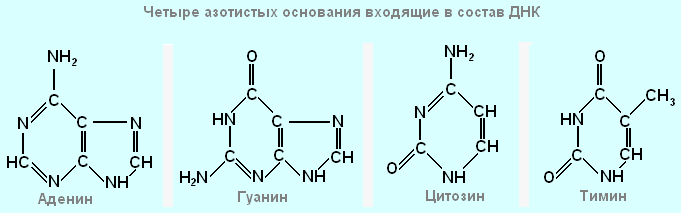
The DNA molecule contains four types of nitrogenous bases: adenine, guanine, cytosine and thymine. They determine the names of the corresponding nucleotides.
9. Determine the composition of the nucleotide.
- phosphoric acid residue, cytidine, carbohydrate
- nitrogenous base, carbohydrate, DNA
- nitrogenous base, carbohydrate, phosphoric acid residue
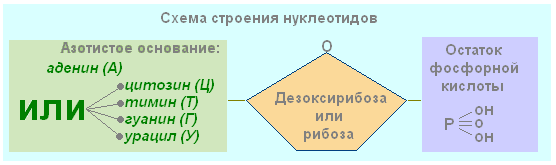
Each nucleotide consists of three components linked by strong chemical bonds. These are nitrogenous base, carbohydrate (ribose or deoxyribose) and phosphoric acid residue.
10. The name of the bond between adenine and thymine in the formation of a double-stranded DNA molecule.
- single
- double
- triple
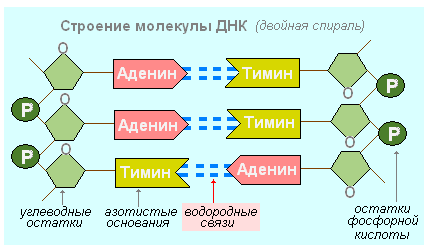
A DNA molecule is a double row of nucleotides, stitched in the longitudinal and transverse directions The framework of its structure is carbohydrates reliably linked by phosphate groups in two chains. Between the chains "ladder" are nitrogenous bases, attracted to each other by weak hydrogen bonds (in the case of adenine-thymine, the bond double).
11. Determine the composition of adenosine triphosphate:
- adenine, uracil, two phosphoric acid residues
- adenine, ribose, three phosphoric acid residues
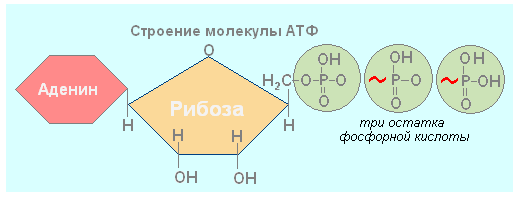
Nucleic acid adenosine triphosphate (ATP) consists of a single nucleotide and contains two high-energy (energy-rich) bonds between phosphate groups. ATP is absolutely essential in every cell, as it plays the role of a biological accumulator - an energy carrier. It is needed wherever energy is stored or released and used, that is, in almost any biochemical reaction, since such reactions occur in every cell almost continuously, each ATP molecule is discharged and recharged, for example, in the human body, on average, once per minute. ATP is found in the cytoplasm, mitochondria, plastids and nuclei.
13. Protein envelope of the virus.
- capsid
- lipid
- there is no right answer
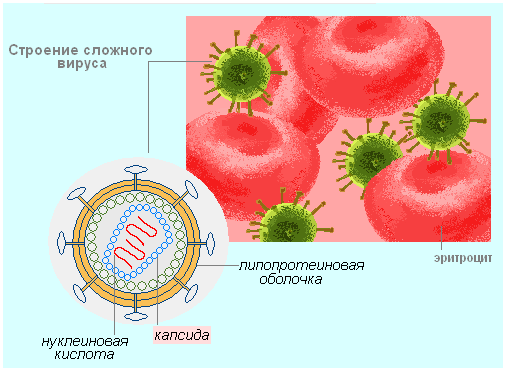
Viruses are arranged very simply. Each viral particle consists of RNA or DNA, enclosed in a protein coat, which is called capsid... The capsid has several functions.
- Protection of the genetic material (DNA or RNA) of the virus from mechanical and chemical damage.
- Determination of the potential for cell infection.
- At the initial stages of cell infection: attachment to the cell membrane, rupture of the membrane and introduction of the genetic material of the virus into the cell.
14. The level represented by the molecules of organic substances found in cells and called biological molecules.
- cellular
- molecular
- organismic
 Molecular the level is represented by molecules of organic substances - proteins, nucleic acids, carbohydrates, lipids. At the molecular level, the role of these important biological compounds in the growth and development of organisms, storage and transmission of hereditary information, metabolism and energy conversion in living cells and other phenomena is being investigated.
Molecular the level is represented by molecules of organic substances - proteins, nucleic acids, carbohydrates, lipids. At the molecular level, the role of these important biological compounds in the growth and development of organisms, storage and transmission of hereditary information, metabolism and energy conversion in living cells and other phenomena is being investigated.
15. Which of the terms is synonymous with the concept of "metabolism"?
- assimilation
- catabolism
- metabolism
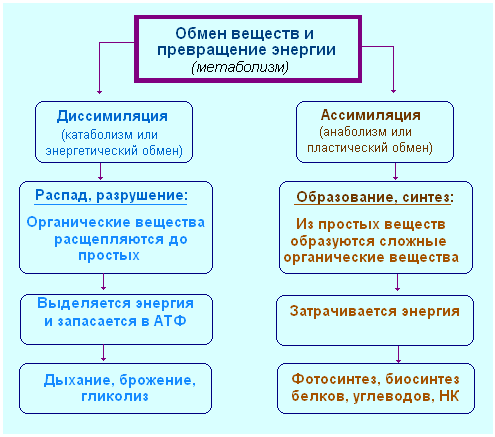
Metabolism ( metabolism) - a set of interrelated processes of synthesis and breakdown of chemicals occurring in the body. Biologists divide it into plastic (anabolism) and energy metabolism (catabolism), which are interconnected. All synthetic processes require substances and energy supplied by cleavage processes. Cleavage processes are catalyzed by enzymes synthesized during plastic metabolism, using the products and energy of energy metabolism. Metabolic reactions in a living cell occur at moderate temperatures, normal pressure and small fluctuations in acidity. Outside living organisms, under such conditions, all chemical reactions of assimilation and dissimilation either could not proceed at all, or would proceed slowly. However, in living organisms, these reactions pass very quickly. This is due to the participation of enzymes in them.
Representatives of the kingdom of viruses are a special group of life forms. They have not only a highly specialized structure, but are also characterized by a specific metabolism. In this article, we will study a non-cellular life form - a virus. What it consists of, how it multiplies and what role it plays in nature, you will learn by reading it.
Discovery of non-cellular life forms
In 1892, the Russian scientist D. Ivanovsky was studying the causative agent of tobacco disease - tobacco mosaic. He found that the pathogenic agent does not belong to bacteria, but is a special form, later called a virus. At the end of the 19th century, microscopes with high resolution were not yet used in biology, so the scientist could not find out what molecules the virus is composed of, and also see and describe it. After the creation of an electron microscope at the beginning of the 20th century, the world saw the first representatives of the new kingdom, who turned out to be the cause of many dangerous and difficult to treat human diseases, as well as other living organisms: animals, plants, bacteria.

The position of non-cellular forms in the taxonomy of living nature
As mentioned earlier, these organisms are combined into a fifth - viruses. The main morphological feature characteristic of all viruses is the absence of cellular structure. Until now, the scientific world does not stop discussions on the question of whether non-cellular forms living objects in the full sense of this concept. After all, all manifestations of metabolism in them are possible only after penetration into a living cell. Up to this point, viruses behave like objects of inanimate nature: they have no metabolic reactions, they do not multiply. At the beginning of the 20th century, a whole group of questions arose before scientists: what is a virus, what does its envelope consist of, what is inside a virus particle? The answers were obtained as a result of years of research and experiments that served as the basis for a new scientific discipline. It originated at the intersection of biology and medicine and is called virology.
Structural features
The expression "all ingenious is simple" directly refers to non-cellular life forms. The virus consists of nucleic acid molecules - DNA or RNA, coated with a protein coat. He does not have his own energy and protein synthesizing apparatus. Without a host cell, viruses have no sign of a living substance: no respiration, no growth, no irritability, no reproduction. For all this to appear, only one thing is required: to find a victim - a living cell, subordinate its metabolism to your nucleic acid, and finally destroy it. As mentioned earlier, the envelope of the virus consists of protein molecules with an ordered structure (simple viruses).

If the envelope also includes lipoprotein subunits, which are actually part of the cytoplasmic membrane of the host cell, such viruses are called complex viruses (causative agents of smallpox and hepatitis B). Often glycoproteins are also part of the surface envelope of the virus. They serve as a signaling function. Thus, both the envelope and the virus itself consist of molecules of the organic component - protein and nucleic acids (DNA or RNA).
How viruses enter living cells
- By merging its shell with the cell membrane (influenza virus).
- By pinocytosis (the causative agent of animal poliomyelitis).
- Through damage to the cell wall (plant viruses).
Reproduction of viruses
The result of the attack of the pathogen on the cell is the combination of DNA or RNA of the virus with its own protein particles. Thus, the newly formed virus consists of nucleic acid molecules coated with ordered protein particles. The host cell membrane is destroyed, the cell dies, and the viruses released from it are introduced into healthy cells of the body.
Reverse reduplication phenomenon
At the beginning of the study of representatives of this kingdom, it was believed that viruses consist of cells, but already the experiments of D. Ivanovsky proved that pathogens cannot be isolated using microbiological filters: pathogens passed through their pores and ended up in a filtrate that retained virulent properties.
Further research established the fact that the virus consists of molecules of organic matter and shows signs of living substance only after its direct penetration into the cell. In it, he begins to multiply. Most RNA viruses reproduce as described above, but some, such as the AIDS virus, induce DNA synthesis in the host cell nucleus. This phenomenon is called reverse replication. Then the m-RNA of the virus is synthesized, and already on it the assembly of viral protein subunits that form its envelope begins.
Features of bacteriophages
What is a bacteriophage - a cell or a virus? What is this non-cellular life form made of? The answers to these questions are as follows: this is a virus that infects exclusively prokaryotic organisms - bacteria. Its structure is quite peculiar. The virus consists of molecules of organic matter and is divided into three parts: the head, the rod (sheath) and the tail filaments. In the front part - the head - there is a DNA molecule. This is followed by a cover that has a hollow core inside. The tail filaments attached to it provide the connection of the virus with the receptor loci of the bacteria. The principle of action of a bacteriophage resembles a syringe. After contraction of the sheath proteins, the DNA molecule enters the hollow rod and is then injected into the cytoplasm of the target cell. Now the infected bacterium will synthesize the DNA of the virus and its proteins, which will inevitably lead to its death.
How the body protects itself from viral infections
Nature has created special protective devices that resist viral diseases plants, animals and humans. The pathogens themselves are perceived by their cells as antigens. In response to the presence of viruses in the body, immunoglobulins are produced - protective antibodies. - thymus, lymph nodes - react to viral invasion and promote the production of protective proteins - interferons. These substances inhibit the development of viral particles and inhibit their reproduction. Both types of defense reactions discussed above are related to humoral immunity. Another form of defense is cellular. Leukocytes, macrophages, neutrophils absorb viral particles and break them down.
The importance of viruses
It's no secret that it is mostly negative. These ultra-small pathogenic particles (from 15 to 450 nm), visible only through an electron microscope, cause a whole bunch of dangerous and intractable diseases of all organisms existing on Earth without exception. So, vital organs and systems are affected, for example, the nervous (rabies, encephalitis, poliomyelitis), immune (AIDS), digestive (hepatitis), respiratory (influenza, adenoinfections). Animals get sick with a lizard, plague, and plants - with various necrosis, spotting, mosaic.
The diversity of representatives of the kingdom is not fully understood. The proof is that new types of viruses are still being discovered and previously uncommon diseases are being diagnosed. For example, in the middle of the 20th century, the Zika virus was discovered in Africa. It is found in the body of mosquitoes, which, when bitten, infect humans and other mammals. Symptoms of the disease indicate that the pathogen primarily affects the central nervous system and causes microcephaly in newborns. People who are carriers of this virus should remember that they pose a potential danger to their partners, since cases of sexual transmission of the disease have been recorded in medical practice.
The positive role of viruses can be attributed to their use in the fight against pest species, in genetic engineering.
In this work, we described what a virus is, what its particle consists of, and how organisms protect themselves from pathogenic agents. We also identified the role non-cellular life forms play in nature.
Contents 1. Molecular level: general characteristics 2. Carbohydrates 2. Carbohydrates. Test your knowledge Test your knowledge 3. Lipids 3. Lipids. Test your knowledge Test your knowledge 4. Protein composition and structure 5. Protein functions 5. Protein functions. Test your knowledge Test your knowledge 6. Nucleic acids 6. Nucleic acids. Test your knowledge Test your knowledge 7. ATP and other organic compounds of the cell 8. Biological catalysts 8. Biological catalysts. Test your knowledge Test your knowledge 9. Viruses 9. Viruses. Test your knowledge Test your knowledge 10. Chapter Contents 11. Literature

Molecular level: general characteristics Molecular level - the initial, deepest level of organization of living organisms Each organism consists of molecules of organic substances in the cell - these are biological molecules Living organisms consist of the same chemical elements as inanimate ones. Currently, more than 100 elements are known, most of them are contained in living organisms The most common in living nature: carbohydrate (C), oxygen (O), hydrogen (H) and nitrogen (N) The basis of all organic compounds is carbon, it enters into bond with many atoms and their groups - forms chains, different in chemical composition, length and shape. Monomers - groups of atoms, relatively simply arranged, which is part of complex chemical compounds Polymer - a chain consisting of numerous links - monomers Biopolymers - polymers that make up living organisms A polymer molecule consists of thousands of interconnected monomers (the same or different) Properties of biopolymers depend on: the structure of monomers the number of monomers the variety of monomers Biopolymers are universal, since they are built according to the same plan for all living organisms.

Molecular level: general characteristics Biopolymers include: proteins carbohydrates nucleic acids Each type of biopolymer is characterized by a certain structure and function: Biopolymers are proteins, they consist of monomers - amino acids, they perform the functions: the main structural material, regulate processes Nucleic acids consist of nucleotides, participate in the transfer of genetic information Carbohydrates consist of monosaccharides, the main energy material of living organisms. Fats are high-molecular organic compounds - the building and energy resource of the organism. The various properties of biopolymers are due to different combinations of several types of monomers. The specific properties of biopolymers are manifested only in a living cell. Continuity between the molecular and the next cellular level is ensured by the fact that biological molecules are the material from which supramolecular cellular structures are formed. protein amino acid nucleic acid nucleotide carbohydrate monosaccharide To content

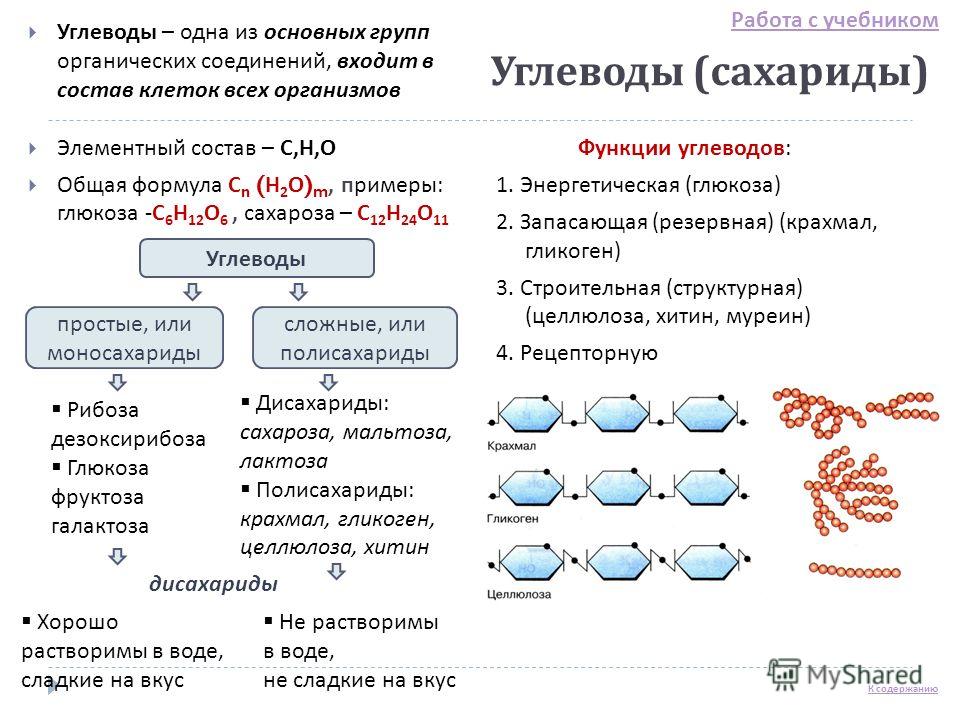
Carbohydrates (saccharides) Carbohydrates - one of the main groups of organic compounds, is part of the cells of all organisms Elemental composition - С, Н, О General formula С n (Н 2 О) m, examples: glucose - С 6 Н 12 О 6, sucrose - С 12 Н 24 О 11 Functions of carbohydrates: 1. Energy (glucose) 2. Storage (reserve) (starch, glycogen) 3. Building (structural) (cellulose, chitin, murein) 4. Receptor Simple carbohydrates, or complex monosaccharides, or polysaccharides Ribose deoxyribose Glucose fructose galactose Disaccharides: sucrose, maltose, lactose Polysaccharides: starch, glycogen, cellulose, chitin Well soluble in water, sweet taste Not soluble in water, not sweet taste disaccharides Working with the textbook To the content
Lipids Lipids - a large group of fat-like substances insoluble in water Most lipids consist of high molecular weight fatty acids and triatomic alcohol glycerol Cells contain from 2-3% to 50-90% Contained in all cells without exception Fats are the simplest and most widespread lipids Elemental composition - С, Н, О Lipid functions: 1. energy 2. storage (fats) 3. water source 4. protective (heat-insulating) 5. promotes buoyancy 6. construction 7. regulatory (hormones). To the content

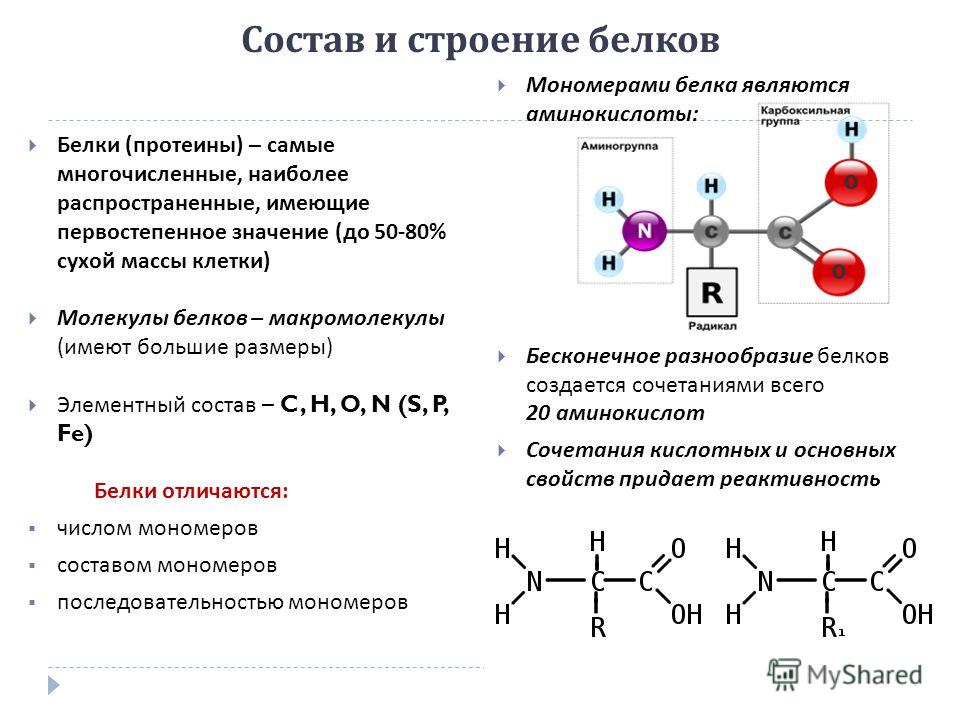
Composition and structure of proteins Proteins (proteins) are the most numerous, the most common, of primary importance (up to 50-80% of dry cell mass) Protein molecules - macromolecules (large) Elemental composition - C, H, O, N (S, P, Fe) Proteins differ: the number of monomers the composition of the monomers the sequence of monomers The monomers of the protein are amino acids: An infinite variety of proteins is created by combinations of only 20 amino acids Combinations of acidic and basic properties impart reactivity
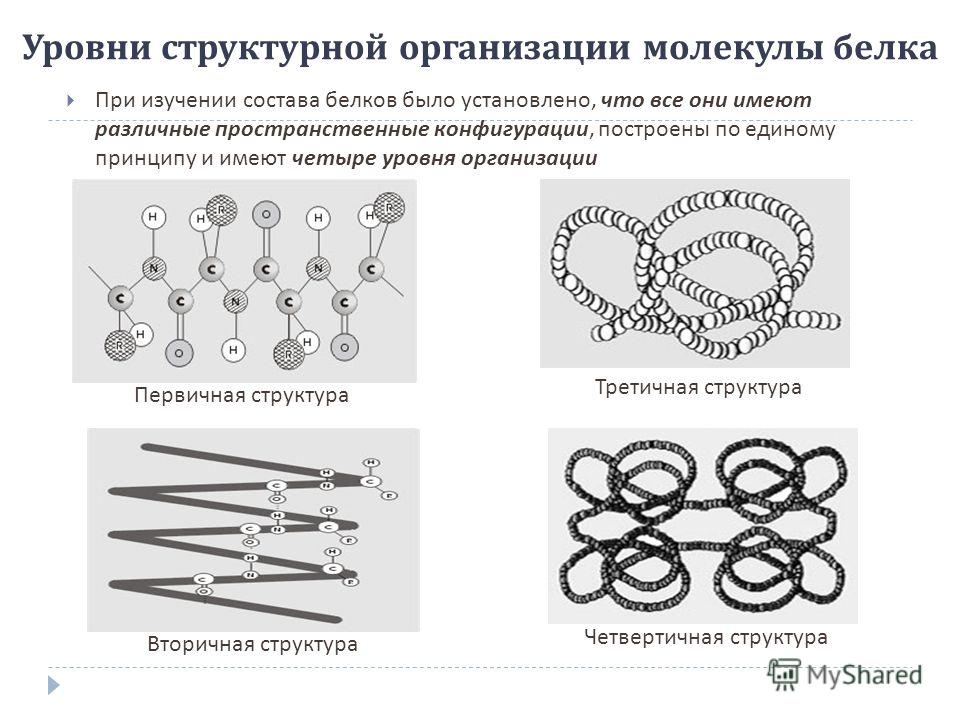
Levels of the structural organization of the protein molecule When studying the composition of proteins, it was found that they all have different spatial configurations, are built according to a single principle and have four levels of organization Primary structure Secondary structure Tertiary structure Quaternary structure
Protein denaturation Protein denaturation Protein denaturation is a loss of their natural properties (solubility) by proteins due to disruption of the spatial structure of their molecules. Denaturation occurs under the influence of: the temperature of radiant energy chemicals, etc. Chemical bonds are destroyed starting from the quaternary structure tertiary secondary primary amino acids This process is partially reversible if destruction proceeded to the primary structure The primary structure determines the structural features of the protein macromolecule. According to their composition, proteins are divided: Simple proteins Complex proteins Consist only of amino acids The composition includes carbohydrates (glycoproteins), fats (lipoproteins), nucleic acids (nucleoproteins) Irreversible denaturation of egg protein To the content

Nucleic acids Nucleic acids are biopolymers in the cell that perform various functions Types of nucleic acids Nucleic acids are biopolymers consisting of monomers - nucleotides Functions of nucleic acids 1. Storage of hereditary information 2. Transport 3. Construction 4. Information. Deoxyribonucleic acid (DNA) Ribonucleic acid (RNA) Each nucleotide consists of: carbohydrate Adenine Thymine Guanine Cytosine Uracil Deoxyribose Ribose r - RNA - ribosomal RNA t - RNA - transport RNA and - RNA - informational, or messenger RNA.
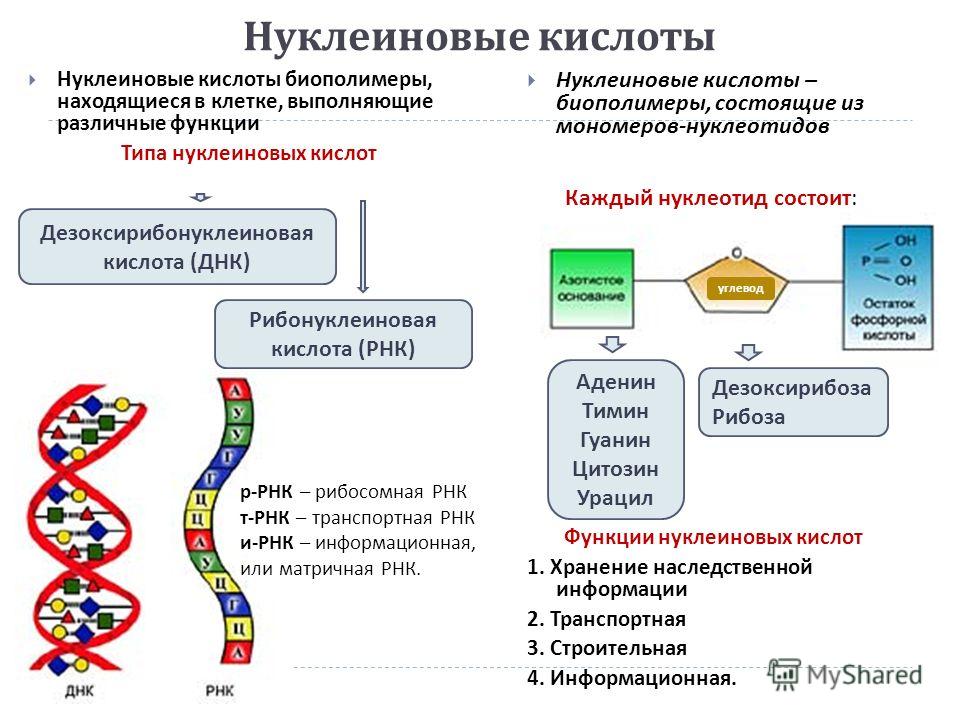
ATP and other organic compounds Adenosine triphosphate (ATP) is a nucleotide consisting of a nitrogenous base of adenine, a carbohydrate ribose and three phosphoric acid residues ATP is an unstable structure Vitamins are complex bioorganic compounds necessary in small amounts of normal life of organisms Some vitamins are synthesized in the body itself, others - come with food Vitamins are designated by letters of the Latin alphabet, are divided into fat-soluble (A, D, E and K) and water-soluble (B, C, PP, etc.) Vitamins play an important role in metabolism - lack or excess in the body violates the physiological functions of B the cell still contains organic substances - intermediate or final products of biosynthesis and decay. 40 kJ Go to content
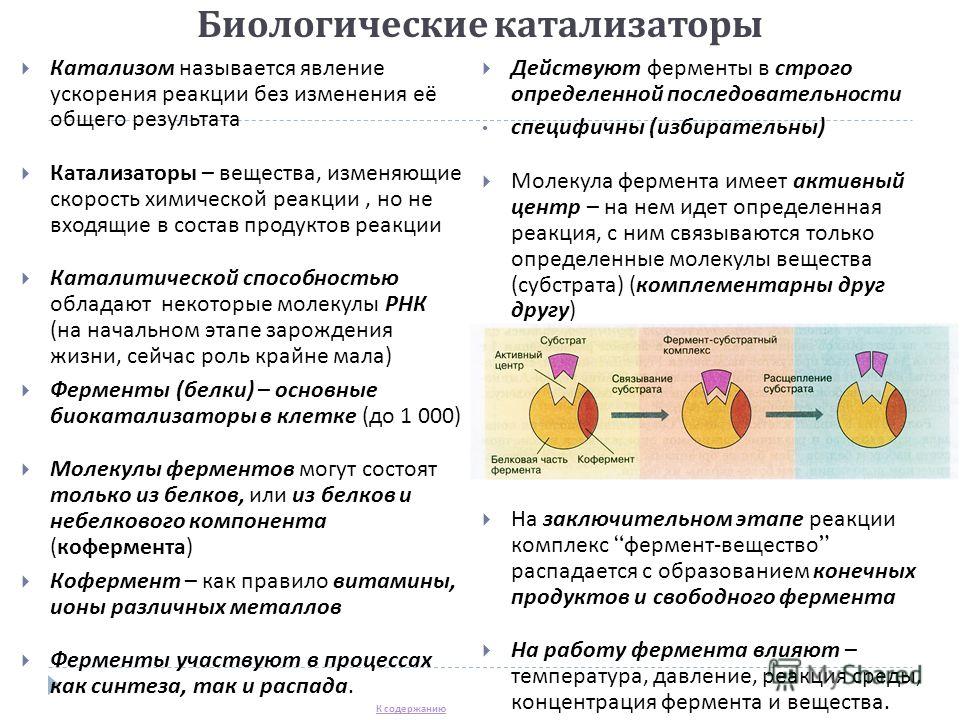
Biological catalysts Catalysis is the phenomenon of accelerating a reaction without changing its overall result Catalysts are substances that change the rate of a chemical reaction, but are not part of the reaction products, some RNA molecules have a catalytic ability (at the initial stage of the origin of life, now the role is extremely small) Enzymes (proteins) - the main biocatalysts in the cell (up to 1,000) Enzyme molecules can consist only of proteins, or of proteins and a non-protein component (coenzyme) Coenzyme - as a rule vitamins, ions of various metals Enzymes are involved in both synthesis and decay processes. Enzymes act in a strictly defined sequence are specific (selective) An enzyme molecule has an active center - a certain reaction takes place on it, only certain molecules of a substance (substrate) bind to it (complementary to each other) At the final stage of the reaction, the enzyme-substance complex decomposes with the formation of final products and free enzyme The work of the enzyme is influenced by temperature, pressure, reaction of the medium, concentration of the enzyme and substance. To the content

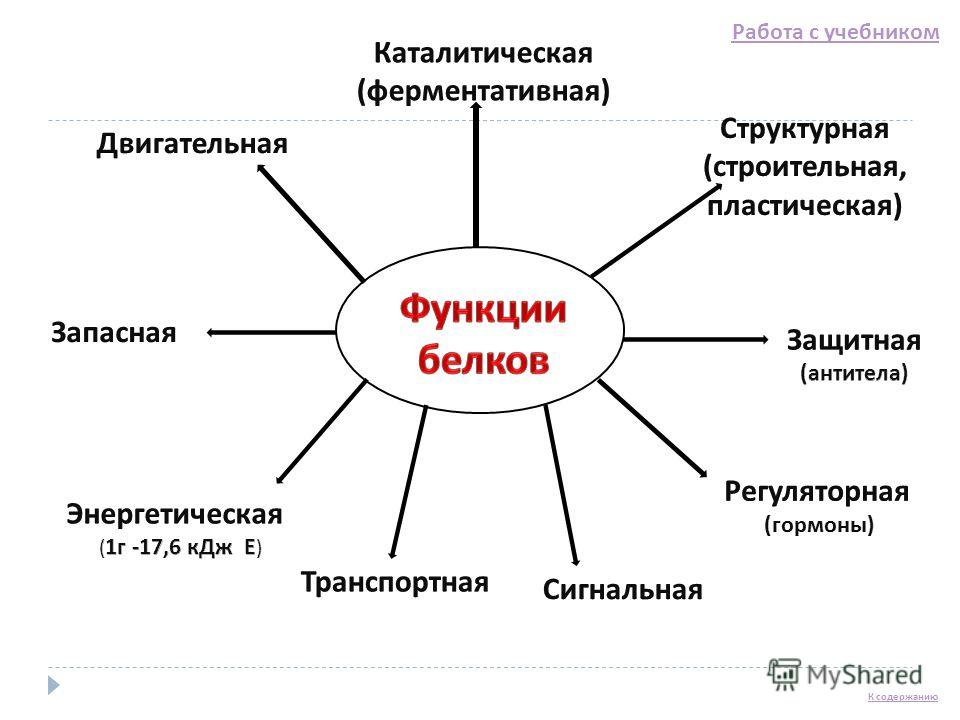
Composition, structure and function of proteins Test your knowledge 1. What substances are called proteins, or proteins? 2. What are the chemical groups of protein monomers? 3. What is the primary structure of a protein? 4. What chemical bonds hold protein configurations? 5. On what grounds are proteins divided into simple and complex? 6. What functions do proteins perform in a living organism? Answer the questions Go to content

Nucleic acids Test your knowledge 1. What is the structure of a nucleotide? 2. What is the structure of the DNA molecule? 3. What is the principle of complementarity? 4. What is common and what are the differences in the structure of DNA and RNA molecules? 5. What types of RNA molecules do you know? What are their functions? Answer the questions Go to content

Biological catalysts Test your knowledge 1. What substances are called catalysts? 2. What role do enzymes play in the cell? 3. Why do most enzymes have high temperature loses its catalytic properties? 4. Why the lack of vitamins can cause disturbances in the vital processes of the organism? Answer the questions Go to content

Viruses Test your knowledge 1. On what basis are viruses classified as living organisms? 2. What features distinguish viruses from other living organisms? 3. What structure do viruses have? 4. What human diseases are caused by viruses? Answer the questions Go to content

P. 20 Working with the textbook 1. Read part of paragraph 1.2, starting with the second paragraph on page Write down in a notebook the main functions of carbohydrates? To the content

P. 24 Working with the textbook 1. Read part of paragraph 1.4 on page 24, starting with the last paragraph 2. Determine what configurations (levels of organization) the protein molecules have, what chemical bonds hold them? 3. Fill in the table: Protein structure Structure characteristic Types of bonds holding structures To content

P. 30 Working with the textbook 1. Read part of paragraph 1.6, starting with the first paragraph on page Define how a DNA molecule differs from an RNA molecule, what are the similarities between these molecules? 3. Fill in the table: Nucleic acid Similarities DNA RNA differences To content


Literature 1. Kamensky A. A. et al., Biology. 9 cl. - M: Bustard, Belyaev D.K. et al., General biology class., M .: Education, Polyansky Yu.I., General biology class., M .: Education, Pugovkin A.P. et al., General biology of grade 9, M .: Education, Encyclopedia for children. Biology, M .: Avanta, 1998

